Background: After an allogenic stem cell transplantation (allo-SCT), patients face the risk of infection, and cytomegalovirus (CMV) is a common pathogen. Severe CMV infections can be fatal, so prophylaxis or early treatment is critical. To manage CMV infections precisely, letermovir is administered for prevention of CMV reactivation. Letermovir also improved patients' overall survival (OS) after allo-SCT in several studies.However, some studies have found that letermovir did not affect OS, and it is still unknown whether letermovir can improve allo-SCT outcome. We investigated whether letermovir had an impact on allo-SCT outcomes.
Methods: We retrospectively studied 443 patients over the age of 18 who received allo-SCT for hematological malignancies from 2004 to 2020 in Anjo Kosei Hospital. The patients were divided into letermovir (n = 56) and non-letermovir (n = 387) groups. Letermovir was administered from day 5 after allo-SCT to day 100 unless severe adverse events were observed. We conducted pp65 antigenemia tests for monitoring CMV reactivation.
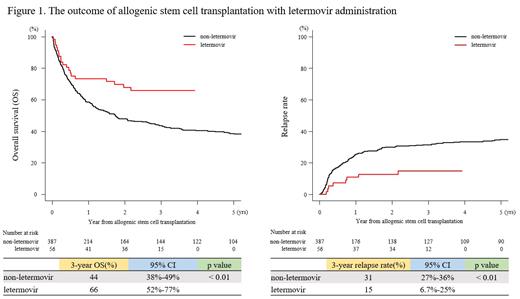
Results: For patients' characteristics, the median age was 49 (range: 18-71) in the letermovir group and 53 (range: 18-72) in the non-letermovir group (p = 0.12). Acute leukemia was the most common disease in both groups. There was no difference in sex (p = 0.77). As stem cell sources, relative peripheral blood cells (PB), unrelated bone marrow, unrelated PB, and cord blood (CB) were used in both groups. In letermovir group, more patients underwent allo-SCT in recent years (p < 0.001) and received anti-thymocyte globulin (ATG) (p < 0.001). The median duration of letermovir administration was 93 days (range: 14-110). The 3-year OS rates were 66% (95%confidence interval [CI]: 52%-77%) and 44% (95%CI: 38%-49%) in the letermovir and non-letermovir groups, respectively (p < 0.01) (Figure 1). Interestingly, non-relapse mortality (NRM) was equivalent, and the relapse rate was 15% (95%CI: 6.7%-25%) vs. 31% (95%CI: 27%-36%) and significantly lower in the letermovir group (p < 0.01) (Figure 1). The cumulative CMV reactivation incidence rates at day 100 were 7.0% (95%CI: 1.1%-26%) and 45% (95%CI: 34%-59%) in the letermovir and non-letermovir groups, respectively (p = 0.037). The GVHD incidence rate in both groups was comparable. Since we observed the difference in the number of ATG administration between the two groups, we conducted the stratification analysis in patients who experienced ATG administration (n = 85). In letermovir (n = 25) and non-letermovir (n = 60) groups, 3-year OS was 84% (95%CI: 63%-94%) and 44% (95%CI: 31%-56%). NRM was equivalent, and the relapse rate was 12% (95%CI: 2.9%-28%) vs. 44% (95%CI: 31%-56%) and significantly lower in the letermovir group (p < 0.01). The cumulative CMV reactivation incidence rates at day 100 were 8.0% (95%CI: 1.3%-23%) and 43% (95%CI: 31%-56%) in the letermovir and non-letermovir groups, respectively (p = 0.077). The incidence rate of GVHD was equivalent between the two groups. In multivariate analysis, letermovir was recognized as a significant factor for OS (hazard ratio [HR]: 0.30, 95%CI: 0.089-0.99, p < 0.05) and relapse rate (HR: 0.27, 95%CI: 0.092-0.80, p < 0.05). In patients who have not received ATG, 3-year OS (48% (95%CI: 30%-64%) vs. 43% (95%CI: 37%-48%), p = 0.86) and relapse rate (19% (95%CI: 7.3%-34%) vs. 30% (95%CI: 25%-35%), p = 0.17) were comparable between letermovir and non-letermovir groups. NRM was also equivalent.
Conclusion: Letermovir administration suppressed the reactivation rate of CMV and improved the outcome of allo-SCT. Interestingly, the letermovir group showed lower relapse rate and this effect was mainly observed in allo-SCT with ATG. Letermovir can possess the positive effect for outcome of the allo-SCT with ATG.
Disclosures
Negishi:NIPPON SHINYAKU CO.: Honoraria. Miyao:Chugai Pharmaceutical: Speakers Bureau; Janssen Pharmaceutical: Speakers Bureau. Ohara:AstraZeneca: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Meiji Seika Pharma Co.: Honoraria. Wakabayashi:AbbVie: Honoraria. Motegi:Novartis International AG: Honoraria; AbbVie: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Chugai Pharmaceutical Co.: Honoraria. Sawa:Sanofi: Honoraria; Janssen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal